|
Computerized System Validation Test Service
바이오써포트는 2002년도에 컴퓨터시스템 밸리데이션 컨설팅 및 기술 시험, 분석 서비스를 시작하여 다음과 같이 CGMP & 21 CFR Part 11, PIC/S GMP가이드라인, EU GMP Annex 11가이드라인, Data Integrity 가이드라인 등에 따라 위험경감(Risk Reduction)을 기반으로 한 컴퓨터시스템 밸리데이션을 실행(시험 및 문서화)하고 컨설팅을 하고 있습니다.
Computerized System Validation (Test, analysis & Documentation)
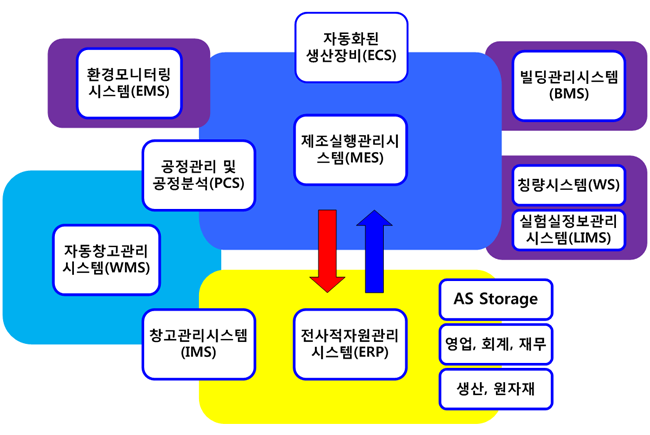
- ERP (Enterprise Resource Planning System)
- MRP (Material Resource Planning System)
- BMS (Building Management System)
- EMS (Environmental Monitoring System)
- MES (Manufacturing Execution System)
- AWS (Automated Weighing System)
- WMS (Warehouse Management System)
- LIMS (Laboratory Information Management System)
- D-QMS(Digital Quality Management System)
- EDMS(Electronic Document Management System)
- Building, Facility, Utility System Control Systems
- Manufacturing Process Control Systems
- IT Infrastructure
- Clinical Trial and Data Management Systems
Computerized System Development
- The Development and Engineering of BMS(Building Management System) for GMP Facility
Computerized System Validation Test, analysis Service
- System Development Management Supporting
- Development of CSV Master Plan
- Computerized System Design Supporting
- 21 CFR Part 11 Assessment & Prioritization
- URS, FS, DS Development
- 21 CFR Part 11 Compliance Protocol, Test and Report
- Data Integrity Compliance Protocol, Test and Report
- DQ, FAT, SAT, IQ, OQ and PQ Protocol, Execution and Report
- System Retirement
- Deviation Resolution
- Retrospective Validation
- Revalidation Protocol, Execution and Report
Regulation Compliance Consulting
- System & Process Gap Analysis
- Computerized System Audit Service
- Electronic Document Mangement
- Electronic Records Retention
- Personnel Training
- SOPs Development and Review
CSV Management Consulting
- CSV Project Management Master Plan
- Risk Assessment
- BCCP (Business Contingency & Continuity Plan)
- IT Infrastructure Development(with our partner)
|
|
 Validation Technology
Validation Technology
 컴퓨터시스템 밸리데이션 기술
컴퓨터시스템 밸리데이션 기술 Validation Technology
Validation Technology
 컴퓨터시스템 밸리데이션 기술
컴퓨터시스템 밸리데이션 기술